An efficient computational model to predict protonation at the amide nitrogen and reactivity along the C–N rotational pathway†
Abstract
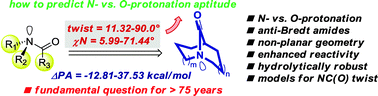
N-Protonation of amides is critical in numerous biological processes, including amide bonds proteolysis and protein folding as well as in organic synthesis as a method to activate amide bonds towards unconventional reactivity. A computational model enabling prediction of protonation at the amide bond nitrogen atom along the C–N rotational pathway is reported. Notably, this study provides a blueprint for the rational design and application of amides with a controlled degree of rotation in synthetic chemistry and biology.

 Please wait while we load your content...
Please wait while we load your content...