Synthesis of pyrroloindolines and furoindolines via cascade dearomatization of indole derivatives with carbenium ion†
Abstract
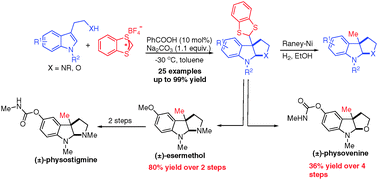
A highly efficient intermolecular cascade dearomatization of substituted indoles with benzodithiolylium tetrafluoroborate has been developed. This reaction provides a novel strategy to synthesise C3 methyl-substituted pyrroloindolines and furoindolines under mild reaction conditions, the utility of which has been demonstrated by the synthesis of esermethol and physovenine in a highly concise manner.

 Please wait while we load your content...
Please wait while we load your content...