An outstanding catalyst for the oxygen-mediated oxidation of arylcarbinols, arylmethylene and arylacetylene compounds†
Abstract
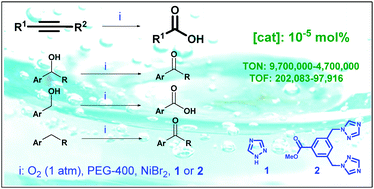
A convenient and sustainable protocol for the aerobic oxidation of benzyl alcohols to carbonyl compounds, based on the use of 1,2,4-triazole-type ligands and nickel(II) bromide, is described. This combination leads to the formation of an exceedingly active, enzyme-like system that allows for other oxidative processes, such as benzylic C–H oxidation and oxygen-mediated cleavage of C–C triple bond, a pioneering procedure for transformation of alkynes into carboxylic acids.

 Please wait while we load your content...
Please wait while we load your content...