Oligonucleotides containing a piperazino-modified 2′-amino-LNA monomer exhibit very high duplex stability and remarkable nuclease resistance†
Abstract
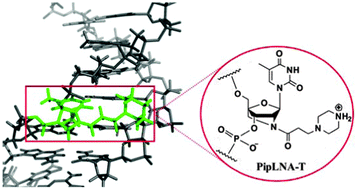
Incorporation of a piperazino-modified 2′-amino-LNA monomer (PipLNA-T) into oligonucleotides conferred very high affinity and base-pairing selectivity towards complementary DNA and RNA strands. Furthermore, one PipLNA-T modification provided a robust nuclease resistance that safeguarded three neighbouring natural nucleosides from 3′-exonucleolytic degradation. These favourable properties render PipLNA-T a promising oligonucleotide modification for various biological applications.

 Please wait while we load your content...
Please wait while we load your content...