Access to polysubstituted indoles or benzothiophenes via palladium-catalyzed cross-coupling of furfural tosylhydrazones with 2-iodoanilines or 2-iodothiophenols†
Abstract
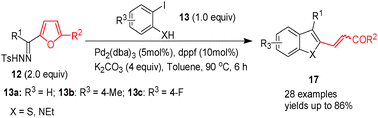
Palladium-catalyzed cross-coupling of furfural tosylhydrazones with 2-iodoanilines or 2-iodothiophenols produces polysubstituted indoles or benzothiophenes, respectively. The reaction proceeds through 2-furylmethylene palladium iodide intermediates, which undergo furan ring opening followed by ring closure to form indoles or benzothiophenes. This reaction will expand the synthetic applications of furfural derivatives.

 Please wait while we load your content...
Please wait while we load your content...