Manganese catalyzed C–H functionalization of indoles with alkynes to synthesize bis/trisubstituted indolylalkenes and carbazoles: the acid is the key to control selectivity†
Abstract
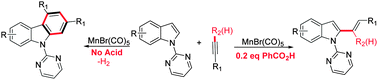
The Mn-catalyzed C–H alkenylation reactions of indole with terminal- and internal-alkynes have been developed. In the presence of a catalytic amount of acid, the procedure efficiently affords bis/trisubstituted indolyl-alkenes in a highly regio- and stereo-selective manner. Without the addition of acid, the reaction undergoes a [2+2+2] cyclization process to give carbazoles with release of hydrogen gas. Notably, the directing pyrimidyl group can be readily removed. Experimental studies reveal that the reaction is initiated by a C–H activation step and the acid is the selectivity controller via a hydrogen transfer process.

 Please wait while we load your content...
Please wait while we load your content...