Thermodynamics of cellulose dissolution in an imidazolium acetate ionic liquid†
Abstract
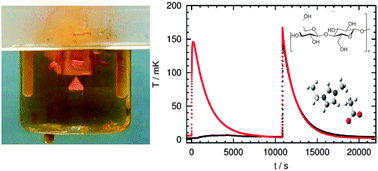
The heat of dissolution of cellulose in one imidazolium acetate ionic liquid was determined experimentally. The value of −132 ± 8 J g−1 indicates that the dissolution is exothermal thus confirming energetically favourable cellulose–ionic liquid interactions but indicating that an increase in temperature does not thermodynamically favour the dissolution process.

 Please wait while we load your content...
Please wait while we load your content...