Reaction of cyclopropenes with a trichloromethyl radical: unprecedented ring-opening reaction of cyclopropanes with migration†
Abstract
The direct addition reaction of chloroform to cyclopropenes under triethylborane-mediated radical reaction conditions to provide trichloromethylcyclopropanes has been developed. In contrast, using dimethylzinc as a radical initiator led to the formation of unconjugated esters via a domino sequence involving the addition of the trichloromethyl radical, rearrangement and ring-opening reactions.
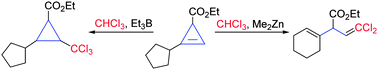

 Please wait while we load your content...
Please wait while we load your content...