Gallium(III)- and calcium(II)-catalyzed Meyer–Schuster rearrangements followed by intramolecular aldol condensation or endo-Michael addition†
Abstract
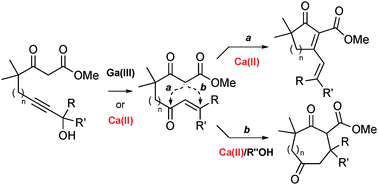
The first gallium- and calcium-catalyzed Meyer–Schuster rearrangements are described. Under substrate control, the incipient conjugated ketones can be trapped intramolecularly by β-keto esters or amides to yield cyclic products after aldol condensation or endo-Michael addition. An interesting additive effect that promotes the latter tandem process with calcium has been found.
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...