Efficient and practical synthesis of enantioenriched 2,3-dihydropyrroles through gold-catalyzed anti-Markovnikov hydroamination of chiral homopropargyl sulfonamides†
Abstract
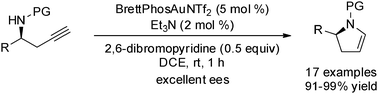
A direct gold-catalyzed 5-endo-dig cycloisomerization of chiral homopropargyl sulfonamides has been developed. A range of enantioenriched 2,3-dihydropyrroles are readily accessed by utilizing this approach. Importantly, this gold-catalyzed cycloisomerization reaction proceeds through an anti-Markovnikov addition by using a catalytic base as the additive, which completely suppresses the undesired dimerization.

 Please wait while we load your content...
Please wait while we load your content...