Oxidative methane activation over yttrium stabilised zirconia
Abstract
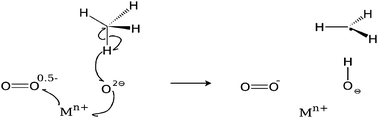
The methane C–H bond is extremely stable, requiring significant energy input in reforming processes. We present a novel mechanism for energetically favourable methane C–H bond breaking over yttrium stabilised zirconia in the presence of oxygen, based on results of Density Functional Theory (DFT) and HSE06 hybrid functional calculations. We argue that this mechanism will be relevant to C–H activation over many metal oxide catalyst materials.

 Please wait while we load your content...
Please wait while we load your content...