Oxidative radical 1,2-alkylarylation of alkenes with α-C(sp3)–H bonds of acetonitriles involving 1,2-aryl migration†
Abstract
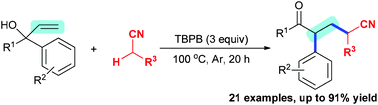
A novel metal-free oxidative 1,2-alkylarylation of unactivated alkenes with the α-C(sp3)–H bonds of acetonitriles for the synthesis of 5-oxo-pentanenitriles is presented. In the presence of TBPB (tert-butyl peroxybenzoate), a variety of α-aryl allylic alcohols underwent the 1,2-alkylarylation reaction with acetonitriles, giving 5-oxo-pentanenitriles in good to excellent yields. This method proceeds via the C(sp3)–H oxidative coupling with the C–C double bond and 1,2-aryl-migration, and represents a new access to acyclic molecules through metal-free oxidative alkene 1,2-alkylarylation.

 Please wait while we load your content...
Please wait while we load your content...