Cu2+ induced formation of Au44(SC2H4Ph)32 and its high catalytic activity for the reduction of 4-nitrophenol at low temperature†
Abstract
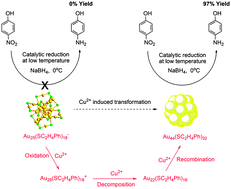
A new nanocluster of atomic precision, Au44(SC2H4Ph)32, is obtained by an oxidation–decomposition–recombination (ODR) process from Au25(SC2H4Ph)18 under mild conditions with high yield (75%). Among the investigated gold nanoclusters, Au44(SC2H4Ph)32 exhibits the highest catalytic activity for the reduction of 4-nitrophenol, especially at low temperature, indicating that the catalytic properties of gold nanoclusters are not only size-dependent, but also structure-sensitive.

 Please wait while we load your content...
Please wait while we load your content...