Metal-free nitrative cyclization of N-aryl imines with tert-butyl nitrite: dehydrogenative access to 3-nitroindoles†
Abstract
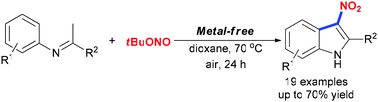
We describe here a new metal-free route for the synthesis of 3-nitroindoles by the nitrative cyclization of N-aryl imines with tert-butyl nitrite. The radical transformation allows the assembly of the indole framework through oxidative cleavage of multi C–H bonds, a nitration, cyclization and isomerization cascade.

 Please wait while we load your content...
Please wait while we load your content...