Abstract
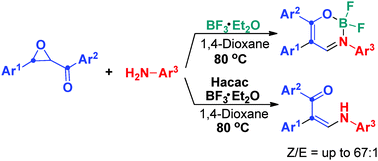
A new domino strategy for selective synthesis of enaminones and their difluoroboron complexes through aryl migration has been developed. The reaction features low-cost and readily accessible starting materials, reliable scalability, and bond-forming efficiency as well as simple one-pot operation, which makes this strategy highly viable for future applications.

 Please wait while we load your content...
Please wait while we load your content...