From “hemoabzymes” to “hemozymes”: towards new biocatalysts for selective oxidations
Abstract
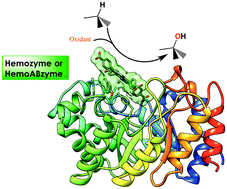
The design of artificial hemoproteins that could catalyze selective oxidations using clean oxidants such as O2 or H2O2 under ecocompatible conditions constitutes a real challenge for a wide range of industrial applications. In vivo, such reactions are performed by heme-thiolate proteins, cytochromes P450, which catalyze the oxidation of substrates by dioxygen in the presence of electrons delivered from NADPH by cytochrome P450 reductase. Several strategies were used to design new artificial hemoproteins that mimic these enzymes. The first one involved the non-covalent association of synthetic hemes with monoclonal antibodies raised against these cofactors. This led to the first generation of artificial hemoproteins or “hemoabzymes” that displayed a peroxidase activity, and in some cases catalyzed the regioselective nitration of phenols by H2O2/NO2 and the stereoselective oxidation of sulfides by H2O2. The second one involved the non-covalent association of easily affordable non-relevant proteins with metalloporphyrin derivatives, using either the “Trojan Horse strategy” or the “host–guest” strategy. This led to a second generation of artificial hemoproteins or “hemozymes”, some of which were found able to catalyze the stereoselective oxidation of organic compounds such as sulfides and alkenes by H2O2 and KHSO5.

 Please wait while we load your content...
Please wait while we load your content...