Palladium/norbornene chemistry: an unexpected route to methanocarbazole derivatives via three Csp3–Csp2/Csp3–N/Csp2–N bond formations in a single synthetic sequence†
Abstract
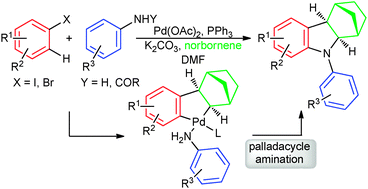
A Catellani reaction terminated by palladacycle amination leading to methanocarbazoles is reported. The outcome is the formation of three different Csp3–Csp2/Csp3–N/Csp2–N bonds through a Heck reaction/C–H activation/double amination cascade in one process.

 Please wait while we load your content...
Please wait while we load your content...