A novel carbamoyl radical based dearomatizing spiroacylation process†
Abstract
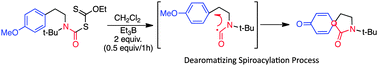
An easy access to novel spirodienonamides based on a dearomatizing spiroacylation process is described for the first time. This process was realized using carbamoylxanthates which were transformed into spirodienonamides containing an acyl-functionalized all-carbon quaternary center.

 Please wait while we load your content...
Please wait while we load your content...