pH- and redox-responsive self-assembly of amphiphilic hyperbranched poly(amido amine)s for controlled doxorubicin delivery†
Abstract
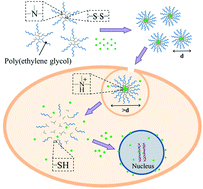
Vinyl-terminated hyperbranched poly(amido amine)s is obtained by Michael addition polymerization of 4-(aminomethyl)piperidine (AMPD) with a double molar N,N-cystaminebis(acrylamide) (BAC). Then an amphiphilic hyperbranched poly(BAC2-AMPD1)-PEG is produced via converting the vinyl groups to amines followed by PEGylation. Transmission electron microscopy (TEM), dynamic light scattering (DLS), and 1H nuclear magnetic resonance (NMR) results indicate that the micelles can be obtained via self-assembly of hyperbranched poly(BAC2-AMPD1)-PEG. Further an anti-cancer drug, doxorubicin (DOX), can be loaded into the micelles. pH- and redox-response of the micelles of hyperbranched poly(BAC2-AMPD1)-PEG without and with DOX are investigated. The results of confocal microscopy and flow cytometry reflect that FITC tagged or DOX loaded micelles of hyperbranched poly(BAC2-AMPD1)-PEG can enter HepG2 and MCF-7 cells, and DOX can be observed in the nucleus of the cells. The cytotoxicity of the micelles without and with DOX is evaluated in HepG2 and MCF-7 cells, and the efficacy to kill the cancer cells is discussed in comparison with free DOX.

 Please wait while we load your content...
Please wait while we load your content...