Hydrophobic–hydrophilic ionic liquids for the extraction and determination of metal ions with water-soluble reagents†
Abstract
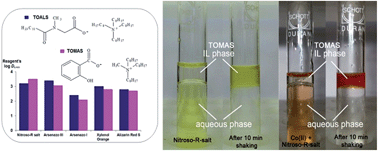
Tetraoctylammonium N-lauroyl sarcosinate and trioctylmethylammonium salicylate are water-immiscible solvents which contain a large amount of dissolved water and may be called “hydrophobic–hydrophilic” ionic liquids (HHILs). We found that several representative water-soluble reagents – Arsenazo I, Arsenazo III, Alizarin Red S, Nitroso-R salt, and Xylenol Orange – are strongly partitioned into the HHIL phase from aqueous solutions over a wide pH range. Metal ions that form complexes with these reagents are extracted efficiently from aqueous solutions. We studied the details of extraction in two representative systems, Sc(III) with Arsenazo III and Co(II) with Nitroso-R salt. A sensitive, selective, and rapid procedure for the extraction-photometric determination of cobalt with HHILs and Nitroso-R salt has been developed.

 Please wait while we load your content...
Please wait while we load your content...