Potentiometric determination of acid dissociation constants of novel biaryl monomers†
Abstract
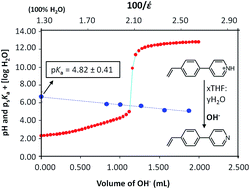
The acid dissociation constants (pKas) of a number of novel polymerisable vinyl biaryl compounds, 4-(4′-ethenylphenyl)-pyridine (M1), 4′-ethenyl-(1,1′-biphenyl)-4-ol (M2), 4′-ethenyl-N,N-dimethyl-(1,1′-biphenyl)-3-amine (M3), 4′-ethenyl-(1,1′-biphenyl)-4-methanol (M4), 4′-ethenyl-N,N-dimethyl-(1,1′-biphenyl)-4-amine (M5), 4′-ethenyl-(1,1′-biphenyl)-4-carboxylic acid (M6), 4′-ethenyl-4-hydroxy-5-methyl-(1,1′-biphenyl)-3-carboxaldehyde (M7) were determined in a mixed solvent (THF–water) potentiometric titration at 25 °C and subsequent extrapolation to pure water via the Yasuda–Shedlovsky method. The acidity and basicity of the compounds in THF–water mixtures was observed to decrease with increasing THF fraction and is attributed to the corresponding decrease in the dielectric constant of the solution. To the best of our knowledge, this is the first reported study of pKa values undertaken for this class of compounds. The biaryls, M1–M7, were prepared by microwave-assisted Suzuki cross coupling of 4-vinylphenyl boronic acid with the appropriate aryl bromide and were custom designed for use as functional monomers in the synthesis of molecularly imprinted polymers.

 Please wait while we load your content...
Please wait while we load your content...