Molybdenum blue photometry method for the determination of colloidal silica and soluble silica in leaching solution
Abstract
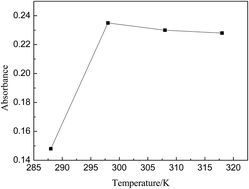
An improved analytical procedure has been described for the spectrophotometric determination of colloidal silica in leaching solution by means of adding fluoride to convert colloidal silica into reactive silica. The method is a variation of the classical molybdenum blue method, and the optimal wavelength, temperature, ratio of H3BO3/NH4F, pH and ammonium molybdate in the colorimetric process has been determined. The pH especially needs to be fairly strictly controlled. The linear equation is established as: C = 3.15956A − 0.51912, and R is more than 0.9992. Its feasibility and reproducibility have been confirmed by ICP-OES; the average relative error is 1.5%. The detection scope of silicon concentration extends almost 1000 times higher in a leaching solution, so the method in this paper can be used to detect leaching samples with a high silica concentration.

 Please wait while we load your content...
Please wait while we load your content...