A new preconcentration procedure to quantify total acid hydrolyzed fluoride in selected beverages and foods by spectrophotometry
Abstract
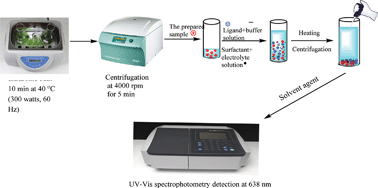
A new micellar-mediated, cloud-point extraction (CPE) method has been developed for the quantification of trace levels of fluoride by means of spectrophotometry. The method is based on the selective ion association of stable anionic complexes, Sn(OH)F2− or Sn(OH)F32− of fluoride with Sn(II) in presence of cationic dye (Nile blue A) at pH 5.0, and its extraction to the micellar-rich phase of nonionic surfactant polyoxyethylene (7.5) nonylphenyl ether (PONPE 7.5) as the extracting agent. Afterwards, the ternary complex formed was spectrophotometrically detected at 638 nm after preconcentration with CPE. Under optimized conditions, the calibration curves were rectilinear in the ranges of 5–25 and 25–360 μg L−1 in the linear region with changing sensitivity. The limits of detection and quantification (LOD and LOQ) (3σblank/m and 10σblank/m) was 1.45 and 4.83 μg L−1, respectively, and the precision (as RSD) for determination of 15, 75, and 150 μg L−1 of fluoride was in the range of 2.35–4.65%. The validity of the method was checked through recovery experiments, independent analysis by potentiometry and analysis of the standard reference material SRM 2695. The developed method was successfully applied to the accurate, sensitive and reliable quantification of total acid hydrolyzed fluoride present in selected beverage and food samples.

 Please wait while we load your content...
Please wait while we load your content...