Quantitative detection of hydroxyl radicals in Fenton system by UV-vis spectrophotometry†
Abstract
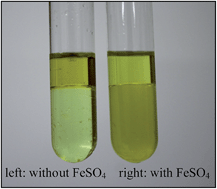
The reason for the prominent error in the hydroxyl radical detection method by UV-vis spectrophotometry with fast blue BB salt (FBBs) as the chromogenic agent was detected, and a modified method was proposed. The simultaneous extraction of FBBs in the diazosulfone extraction process was proven to have a distinct influence on the test results. In the modified method, FeSO4 was adopted to limit the extraction of FBBs by a toluene/butanol extraction agent, which sharply decreased the influence of FBBs on diazosulfone detection. The FBBs amount and extraction time played an important role in its extraction. The appropriate FBBs/MSIA ratio was 50, and the reasonable extraction time was 300 s for the modified method. ˙OH concentration in the directional decomposition of the Fenton system was detected. The test error was about 7%. This method is quite significant to test ˙OH quantificationally to better understand Fenton reaction mechanisms and promote the generation of ˙OH.


 Please wait while we load your content...
Please wait while we load your content...