Determination of very low amounts of free copper and nickel ions in beverages and water samples using cloud point extraction assisted by silver nanoparticles
Abstract
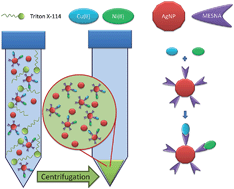
Silver nanoparticles (AgNPs), functionalized with the sodium salt of 2-mercaptoethanesulfonic acid (MESNA), can be used in a cloud point extraction process with Triton X-114 to transfer low amounts of copper and nickel ions to the surfactant-rich phase. Both metals are then measured in the coacervate by electrothermal atomic absorption spectrometry. For a 20 mL aqueous phase containing a low concentration (100 μg L−1) of the functionalized AgNPs and 0.15% Triton X-114, an enrichment factor of 510 is achieved. The detection limits are 2.4 and 2.1 ng L−1 for copper and nickel, respectively. The reliability of the procedure is verified by analyzing three water samples with certified copper and nickel contents. The procedure is also applied for the determination of these analytes in wine and beer samples. The results indicate that only free or labile ionic copper and nickel species are transferred to the coacervate, thus providing a way for rapid fractionation of these compounds without the need for a previous solid phase extraction step.

 Please wait while we load your content...
Please wait while we load your content...