Accessible glyco-tripod amphiphiles for membrane protein analysis†
Abstract
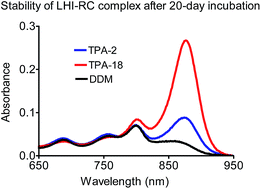
Membrane protein manipulation is known to be an extremely challenging task, mainly because of incompatibility between the hydrophobic surface area of proteins and the hydrophilic character of an aqueous medium. To avoid protein degradation resulting from this incompatibility, detergents are used as essential tools in the study of membrane proteins. However, traditional detergents have a limited ability to stabilize the native conformation of membrane proteins. This study introduces a novel tripod amphiphile that can be prepared efficiently from a commercially available compound. The new agent proved effective for the long-term stability of a multi-subunit superassembly, a membrane protein sensitive to denaturation.

 Please wait while we load your content...
Please wait while we load your content...