Utilizing LC-MS/MS to provide adaptable clinical bioanalytical support for an extended half-life bioactive peptide fused to an albumin-binding domain antibody
Abstract
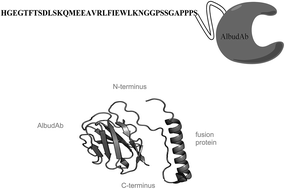
Bioactive peptides are often unstable in the body leading to short half lives and requiring frequent dosing intervals. Linking these peptides to moieties such as albumin, fatty acids and polyethylene glycol has been shown to extend the half-lives of various therapeutics allowing less frequent dosing regimens. In this study the GLP-1 receptor agonist, therapeutic under investigation (GSK2374697), was a bioactive peptide (exendin-4) that was fused to an albumin-binding domain antibody (AlbudAb™) to increase the half-life of the therapeutic. However, developing selective quantitative methods for these molecules to provide a complete understanding of the pharmacokinetic (PK) properties using immunoassay, has proved to be challenging. Methods utilizing LC-MS/MS for the determination of GSK2374697 in human plasma were based on the selection and quantification of two surrogate peptides after enzymatic digestion using either Lys-C or trypsin. These methods were validated and used for the analysis of clinical samples from a first time in human (FTIH) study. Method validation data for both surrogate peptides indicate that the methods are rugged, accurate, precise and well suited for support of regulated clinical studies. The pharmacokinetic results obtained from the two surrogate peptides indicate that the peptide derived from the bioactive portion of the molecule has a much shorter terminal half-life than the peptide derived from the AlbudAb portion of the molecule. Development of assays for these multiple molecular fragments allowed for the accurate quantification and integrity of the molecule from different binding regions illustrating different AUCs and half lives.

 Please wait while we load your content...
Please wait while we load your content...