Simultaneous determination of four main impurities in cefuroxime lysine by ultra fast liquid chromatography-tandem mass spectrometry: application to the analysis of products in stability testing
Abstract
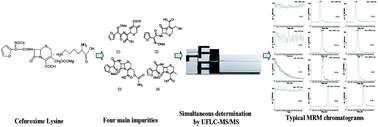
Cefuroxime lysine (CL), a new kind of cefuroxime salt with the merits of better water solubility and causing less irritation of the veins, has been invented in China. In order to better control the drug quality, a fast, sensitive and high throughput ultra fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS) method has been developed and validated for the simultaneous determination of four main impurities in cefuroxime lysine. Chromatographic separation was performed on a Shim-pack XR-ODS column (75 mm × 3.0 mm, 2.2 μm) using a mobile phase composed of acetonitrile–0.1% formic acid (70 : 30, v/v) within a run time of 6.0 min. The detection of the analytes was performed on a 4000Q UFLC-MS/MS system with a turbo ion spray source in the positive ion and multiple reaction-monitoring (MRM) mode. For the assay of the four impurities, all the linear regressions showed good linear relationships (r > 0.9990). The limits of detection (LOD) were 2.5, 5.0, 3.0 and 4.0 ng mL−1, respectively, and the limits of quantification (LOQ) were 8.4, 16.5, 9.9 and 13.2 ng mL−1, respectively. The precision and repeatability were evaluated, and relative standard deviation (RSD) values were all less than 1.7%. The recoveries were between 98.8% and 101.3%. The validated method was successfully applied to simultaneously determine the four main impurities in batches of cefuroxime lysine samples in stability testing.

 Please wait while we load your content...
Please wait while we load your content...