Capabilities of several phosphonium ionic liquids for arsenic species determination in water by liquid–liquid microextraction and electrothermal atomic absorption spectrometry
Abstract
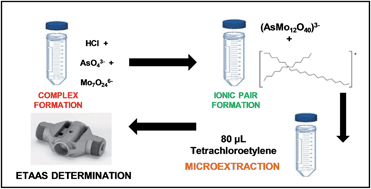
The capabilities of several phosphonium-ionic liquids (PILs) to form ion-pairs with a complex obtained by reaction of arsenate species with molybdate were evaluated. Phosphonium-ILs containing the tetradecyl(trihexyl)phosphonium cation but different anions (dicyanamide and decanoate) and tributyl(methyl)phosphonium methylsulphate IL were studied. The size, polarity and localization of charges in PIL cations were shown to influence their capability to form ion-pairs with the arsenomolybdate (AsMo12O403−) complex and to extract As(V). The performance of PILs was compared with that of a widely used ion-pairing reagent, cetyltrimethylammonium bromide (CTAB). Finally, the IL tetradecyl(trihexyl)phosphonium dicyanamide was chosen to develop a liquid–liquid microextraction (LLME) procedure using only 80 μL of tetrachloroethylene as the extractant. The organic phase was directly injected into the graphite furnace of an electrothermal atomic absorption spectrometer (ETAAS) for As determination. An extraction efficiency of 100% and a sensitivity enhancement factor of 130 were obtained with 5 mL of sample. The detection limit was 1.9 ng L−1 and the relative standard deviation for six replicate measurements of 1.0 μg L−1 for As was 4.9%, 5.0% and 5.1% for As(V), As(III) and organic As species, respectively.
- This article is part of the themed collection: Analytical Chemistry in South America

 Please wait while we load your content...
Please wait while we load your content...