Qualitative and quantitative evaluation of ginkgo terpene lactone raw material by HPLC/Q-TOF MS combined with HPLC-DAD-ELSD
Abstract
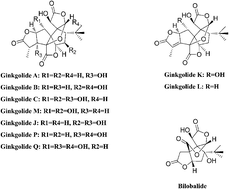
Ginkgo terpene lactone raw material (GTL) is extracted and purified from the dry leaves of Ginkgo biloba L., and it is the raw material in ginkgo diterpene lactone meglumine injections for treating patients suffering from mild to moderate cerebral infarction (manufactured by Jiangsu Kanion Pharmaceutical Co., Ltd., Lianyungang, Jiangsu, China). In the present study, an efficient strategy was developed for the characterization and identification of chemical components in GTL using high performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry (HPLC/Q-TOF MS/MS) combined with online valve switching. The structures of compounds were elucidated based on the accurate mass measurements, the fragmentation patterns of standards and the fragment ion at m/z 57.0700 of the tert-butyl group. As a result, 12 ginkgo terpene lactones were screened and identified from GTL, including ginkgolide A, B, C, J, M, K, L, Q, and P as well as bilobalide, one isomer of ginkgolide C and one isomer of ginkgolide A. Furthermore, four main ginkgolides (ginkgolide A, B, C and K) were simultaneously determined using a high performance liquid chromatograph coupled with a diode-array detector and an evaporative light scattering detector method (HPLC-DAD-ELSD) for the first time. The quantitative analytical method was fully validated with respect to linearity (r2 > 0.998), sensitivity, precision, accuracy (the recovery was between 99.7% and 100.4%), and robustness. Ten batches of samples were detected by HPLC-DAD-ELSD, indicating that the proposed approach was applicable for the evaluation of GTL quality.

 Please wait while we load your content...
Please wait while we load your content...