Comparing equilibrium and kinetic protein unfolding using time-resolved electrospray-coupled ion mobility mass spectrometry
Abstract
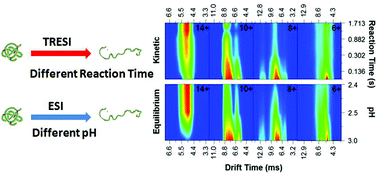
Protein unfolding intermediates are thought to play a critical role in conformational pathogenesis, acting as a ‘gateway’ to inactivation or pathogenic aggregation. Unfolding intermediates have long been studied either by populating partially-folded species at equilibrium using incresingly denaturing conditions, or by transiently populating ‘kinetic’ intermediates under fully denaturing conditions using a time-resolved approach (e.g. stopped-flow fluorescence). However, it is not clear that the folding intermediates populated under equilibrium conditions are comparable to intermediates transiently populated in kinetic experiments. In this work, we combine time-resolved electrospray (TRESI) with travelling wave Ion Mobility Spectrometry (IMS) for the first time to directly compare equilibrium and kinetic unfolding intermediates of cytochrome c. Our results show a high degree of correlation between all species populated under these substantially different regimes.
- This article is part of the themed collection: Ion Mobility Mass Spectrometry

 Please wait while we load your content...
Please wait while we load your content...