Evaluation of photoluminescence quenching for assessing the binding of nitroaromatic compounds to a tyrosyl bolaamphiphile self-assembly†
Abstract
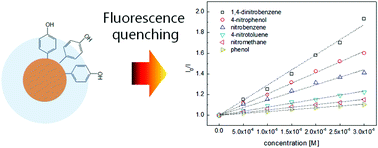
Quenching of a fluorophore is significantly influenced by the molecular structure of the quencher. In this study, photoluminescence quenching by nitroaromatic compounds was evaluated to assess the molecular interaction between nitroaromatic molecules and the photoluminescent tyrosyl bolaamphiphile self-assembly, a nanoscale optical photoluminescent probe. Both the aromatic structure and hydrophilic functional groups of the nitroaromatic quencher molecules significantly enhanced the binding of quencher molecules to the photoluminescent probe. UV-vis spectroscopy supported the non-covalent molecular association of aromatic stacking, which significantly increased the quenching efficiency compared to an aliphatic compound. The hydrophilic groups of the nitroaromatic compounds also enhanced the photoluminescence quenching, because of the hydrophilic nature of the phenol moiety. Energy levels of the photoluminescent probe and quencher molecules, along with molecular interactions, were investigated to explain the quenching mechanism. Density functional theory (DFT) calculation was performed to provide the energy levels and charge density of the nitroaromatic compounds. The information presented in this study regarding the structural effect of a quencher molecule on the photoluminescence quenching of the photoluminescent probe will be useful in designing binding motifs of future photoluminescent probes.

 Please wait while we load your content...
Please wait while we load your content...