Enantioseparation characteristics of tadalafil and its intermediate on chitin derived chiral stationary phases
Abstract
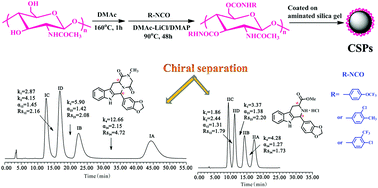
Due to the low solubility and swelling properties of chitin bis(arylcarbamate) in most organic solvents, the chiral stationary phases (CSPs) prepared from chitin derivatives can be analyzed with a wide range of solvents. In order to develop new CSPs of chitin derivatives with halogen groups, chitin was derivatized with three different phenyl isocyanates to obtain chitin bis(4-trifluoromethoxyphenylcarbamate), chitin bis(3-chloro-4-methylphenylcarbamate) and chitin bis(4-chloro-3-trifluoromethylphenylcarbamate). Then, the three chitin derivatives were coated on macroporous 3-aminopropyl-functionalized silica gel to obtain three CSPs 1–3 respectively. These CSPs showed good enantioseparation capabilities towards tadalafil and its intermediate. Therefore, these CSPs are potentially applied for the enantioseparation and determination of tadalafil and its intermediate. It was also found that the retention times of some enantiomers were prolonged in the presence of their diastereoisomers or structural analogues and as a result, the resolution was improved to some extent. Hence, in the enantioseparation of a compound with two chiral centers, the resolution is hopefully improved by adding a diastereoisomer or a structural analogue.

 Please wait while we load your content...
Please wait while we load your content...