A novel water-soluble sulfonated porphyrin fluorescence sensor for sensitive assays of H2O2 and glucose†
Abstract
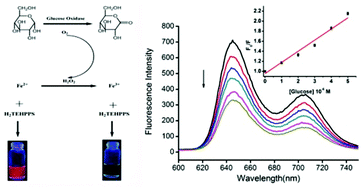
Water soluble porphyrins have many perfect analytical figures of merit. A water-soluble sulfonated porphyrin (H2TEHPPS) was used to build a novel platform for sensitive assays of hydrogen peroxide and glucose based on the different effects of Fe2+ and Fe3+ on H2TEHPPS. H2O2 or Fe2+ alone cannot induce a fluorescence change in H2TEHPPS, but Fe3+ can quench the fluorescence of H2TEHPPS significantly. Interestingly, glucose is oxidized to gluconolactone by GOD and generates an equivalent hydrogen peroxide, and the produced H2O2 also oxidizes Fe2+ to Fe3+ and causes the fluorescence quenching of H2TEHPPS. According to this, a sensitive sensor for hydrogen peroxide and glucose has been demonstrated, which can determine H2O2 and glucose in a relative simple and sensitive way. The detection limits were 1.3 × 10−7 M and 3.2 × 10−7 M for H2O2 and glucose, respectively. In addition, the glucose in serum samples was determined successfully using this sensing platform. It is also noteworthy that H2O2 can be released in almost all oxidations catalyzed by oxidases, which suggests that this newly proposed H2O2 probe can be readily extended to sense other oxidases and their specific substrates.

 Please wait while we load your content...
Please wait while we load your content...