Evaluation of synthesized lipid tethered ligands for surface functionalization of polypropylene capillary-channeled polymer fiber stationary phases†
Abstract
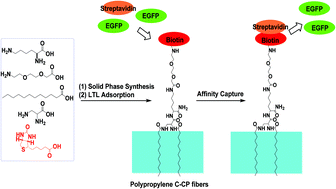
Polypropylene (PP) capillary-channeled polymer (C-CP) fibers have been used in this laboratory as stationary phases for high performance liquid chromatography and solid phase extraction of proteins. Greater selectivity has been realized through the functionalization of the PP fibers through the physical adsorption of commercially available head group-modified poly(ethylene glycol) lipids (PEG-lipids), where the head group is chosen to affect affinity separations. We refer to this general surface modification methodology as lipid tethered ligands (LTLs). In this study, LTLs were synthesized by solid phase synthesis. In comparison to the commercial PEG-lipids, the synthesized LTLs contain no chemically labile phosphate groups. Instead of an ester linkage in the commercial lipids, amide functionality was used in the synthesized LTLs to attach the lipids and ligands. By use of fluorescence imaging of FITC-labeled LTLs, the synthesized LTL was shown to be superior to the commercial LTL in terms of the adsorption efficiency to PP C-CP fibers, the resistance to solvent wash from the PP C-CP fibers, and their chemical stability under acidic, neutral and basic conditions. The PP C-CP fibers functionalized with a synthesized LTL that was biotinylated at the head group are shown to be capable of capturing streptavidin from E. coli cell lysate more efficiently than the PP C-CP fibers functionalized with the commercial biotinylated PEG-lipid. The functionalization of PP C-CP fibers with the synthesized LTLs is a simple, but highly efficient, method to generate novel stationary phases with a variety of functionalities for solid phase extraction and liquid chromatography.

 Please wait while we load your content...
Please wait while we load your content...