Safety biomarkers for drug-induced liver injury – current status and future perspectives
Abstract
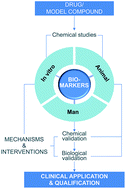
Drug-induced liver injury (DILI) is a major contributor to attrition within drug development, causing a significant patient morbidity and mortality. Prediction of clinical DILI from in vitro and in vivo data remains difficult. Currently utilized hepatic injury biomarkers often lack sensitivity and specificity to indicate that a patient is progressing toward overt DILI; thus, new biomarkers are needed. Currently, ‘Hy's law’, reflecting ALT and bilirubin increases, represents the gold standard model to predict serious DILI and the standard for novel DILI biomarkers to surpass. Pre-clinical and clinical studies have shown the utility of biomarkers such as cytokeratin-18, high mobility group box-1, glutamate dehydrogenase, microRNA-122 and HLA-associated genotypes as sensitive and translational biomarkers of DILI. Qualification of these biomarkers requires additional data, including inter- and intra-subject variability, impact of gender, age, and diurnal variation in healthy volunteers. Additionally, the utility of these biomarkers to identify idiosyncratic DILI and the prediction of serious DILI from benign elevations in ALT activity remain to be assessed. These challenges are the current focus of the Predictive Safety Testing Consortium (PSTC) in the US and the IMI Safer and Faster Evidence based Translation (SAFE-T) consortium in Europe. However, in the near future, these proposed biomarkers are likely to retain their exploratory status as formal biomarker qualification is lengthy and challenging. A more intelligent use of currently used markers, such as ALT activity, in the meantime can bridge the gap between novel biomarkers being used in the experimental context to formal qualification. The current status of novel DILI safety biomarkers with clinical potential and their use alongside established markers will be discussed in the context of advancing fundamental mechanistic drug safety science.

 Please wait while we load your content...
Please wait while we load your content...