Impact of N-substitution of a carbazole unit on molecular packing and charge transport of DPP–carbazole copolymers†
Abstract
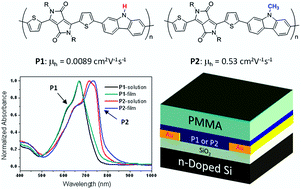
Two diketopyrrolopyrrole (DPP)–carbazole (Cz) based π-conjugated copolymers, PDBTCz-H (P1) and PDBTCz-Me (P2), were designed and synthesized to study the effects of N-substitution of the carbazole unit on the molecular ordering, main chain conjugation, and charge transport properties of these polymers. It was found that the existence of hydrogen bonding interaction between the N–H group in the carbazole unit and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in the DPP unit has a significant impact on the UV absorption, crystallinity, thin film morphology, as well as charge transport characteristics of P1. The hydrogen bonding is a very competitive force with the π–π stacking interaction, leading to the more twisted backbone structure and poorer molecular ordering of P1 in the solid state. Although the crystallinity of the P1 thin films could be somewhat improved by thermal annealing, the polymer main chains of P1 remain rather twisted and less conjugated in comparison with P2. The poorer main chain conjugation of P1 caused by the hydrogen bonding led to a dramatic drop in charge transport performance in organic thin film transistors (OTFTs). The highest hole mobility achieved for P1 is 8.9 × 10−3 cm2 V−1 s−1, which is almost two orders of magnitude lower than that of P2 (0.53 cm2 V−1 s−1).
O group in the DPP unit has a significant impact on the UV absorption, crystallinity, thin film morphology, as well as charge transport characteristics of P1. The hydrogen bonding is a very competitive force with the π–π stacking interaction, leading to the more twisted backbone structure and poorer molecular ordering of P1 in the solid state. Although the crystallinity of the P1 thin films could be somewhat improved by thermal annealing, the polymer main chains of P1 remain rather twisted and less conjugated in comparison with P2. The poorer main chain conjugation of P1 caused by the hydrogen bonding led to a dramatic drop in charge transport performance in organic thin film transistors (OTFTs). The highest hole mobility achieved for P1 is 8.9 × 10−3 cm2 V−1 s−1, which is almost two orders of magnitude lower than that of P2 (0.53 cm2 V−1 s−1).

 Please wait while we load your content...
Please wait while we load your content...