Repeatable fluorescence switcher of Eu3+-doped CeO2 nanorods by l(+)-ascorbic acid and hydrogen peroxide†
Abstract
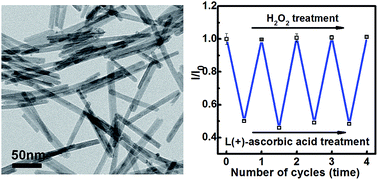
Inorganic phosphors of Eu3+-doped CeO2 nanorods were synthesized by a hydrothermal method, followed by high temperature calcination. The phosphors with various doping concentrations of Eu3+ exhibited similar rod-like morphology. These phosphors with different Eu3+ contents can be effectively excited at 340 nm due to the charge transfer from O2− to Ce4+, and display orange and red emissions of Eu3+. It was found that the fluorescence intensity of Eu3+-doped CeO2 nanorods was exponentially decreased with increase in the concentrations of L(+)-ascorbic acid, resulting from the chemical reduction of Ce4+ into Ce3+ and the subsequent excitation cut-off of O2− to Ce4+. The fluorescence intensity of reduced phosphors was restored to the original intensity reversibly by adding hydrogen peroxide, which oxidized Ce3+ into Ce4+. The evolution in fluorescence intensity was consistent with surface Ce3+ fractions of Eu3+-doped CeO2 nanorods, which were 22.0% for as-synthesized phosphors, 34.6% for reduced phosphors and 23.2% for restorative phosphors oxidized by H2O2, respectively. The quantitative and repetitive fluorescence switching of Eu3+-doped CeO2 nanorods by adding L(+)-ascorbic acid and hydrogen peroxide alternatively indicates their potential applications as fluorescence switching devices and chemical or biological sensors for detection and monitoring.

 Please wait while we load your content...
Please wait while we load your content...