Abstract
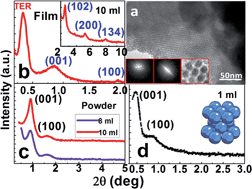
This is the first report on the critical nature of nanolattice formability of different particle sizes (∼4–10 nm) of monodispersed nickel nanoparticles. They exhibit strikingly hexagonal close-packed (hcp) nanolattices without extra forces whenever trioctylphosphine (TOP) is one of the surfactant(s). This clearly establishes the unique role of nanolattice formability of TOP. The c/a ratios are interestingly identical to those of atomic lattices. An attempt has also been made to explain them based on the balanced attractive and repulsive forces of the surfactant-generated cation–anion pairs on the surface of the nanoparticles. The present findings therefore will provide a far-reaching vista for the fabrication of varieties of natural nanolattices and their understanding on applications in a new paradigm.

 Please wait while we load your content...
Please wait while we load your content...