Three-colored electrochromic lithiated vanadium oxides: the role of surface superoxides in the electro-generation of the red state†
Abstract
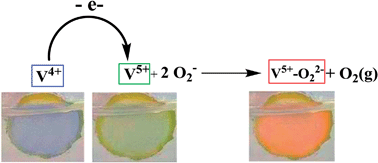
Lithiated vanadium oxides with polyelectrochromic behaviour are reported here for the first time. Electrochrome was obtained by means of a simple and solvent-free solid state reaction. Clear XPS evidence indicates that the electro-generation of the red color is due to the dismutation of surface-adsorbed O2− species with subsequent formation of red colored V5+–O22− complexes. The reaction is switched electrochemically by oxidation of the surface V4+ centers.

 Please wait while we load your content...
Please wait while we load your content...