A novel, warm, white light-emitting phosphor Ca2PO4Cl:Eu2+, Mn2+ for white LEDs†
Abstract
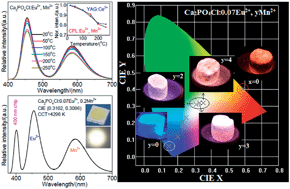
A series of single-phase phosphors, Ca2PO4Cl:Eu2+, Mn2+, have been successfully synthesized by a solid-state method and their photoluminescence properties have been investigated. Ca2PO4Cl:Eu2+, Mn2+ can be excited at wavelengths between 250 and 450 nm, which is within the range of the ultraviolet light-emitting diode (LED). As a result of fine tuning the emission composition of Eu2+ and Mn2+ ions, warm white light can be realized by combining the emission in a single host lattice under excitation by ultraviolet light. Efficient resonant energy transfer from Eu2+ to Mn2+ ions has been demonstrated to be a dipole–quadrupole mechanism in Ca2PO4Cl, and the energy transfer efficiency increases with increasing Mn2+ concentration, confirmed by luminescence spectra and fluorescence decay times. The energy transfer efficiency and critical distance have also been calculated. A warm, white LED has been fabricated using the single-phase white-emitting phosphor, Ca2PO4Cl:0.07Eu2+, 0.2Mn2+, pumped by a 400 nm LED chip. Our results give CIE chromaticity coordinates for the white LEDs as (0.3102, 0.3096), and a correlated color temperature of 4296 K. Ca2PO4Cl:Eu2+, Mn2+ is therefore able to serve as a potential, warm, white emitting material for white LEDs.

 Please wait while we load your content...
Please wait while we load your content...