Photoluminescence activity of Ba1−xCaxTiO3: dependence on particle size and morphology
Abstract
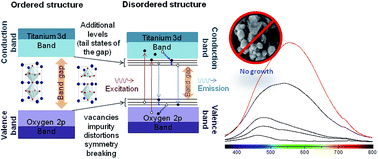
It was shown that the higher photoluminescence emission of Ba1−xCaxTiO3 titanates is associated with the substitution of Ba by Ca, for the composition x = 0.75, which interferes with structural ordering, morphology and particle growth. Although the XRD data of this sample showed that the crystals do not exhibit long range order, FTIR, Raman and XPS confirmed the presence of Ti–O bonds in this sample, indicating the particle nucleation of titanates, but without growth and maintaining only short range order. These nanoparticles show perturbations in the symmetry of the unit cell, causing structural distortions, which result in changes in their electronic structure of atoms, destabilizing the whole lattice. This fact gives rise to points of intrinsic defects, changing the intermediate levels in the band gap, subsequently adding to the population of charge carriers and, consequently, radiative recombination with higher photoluminescence emission.

 Please wait while we load your content...
Please wait while we load your content...