Two-photon absorption in a conformationally twisted D–π–A oligomer: a synergic photosensitizing approach for multiphoton lithography†
Abstract
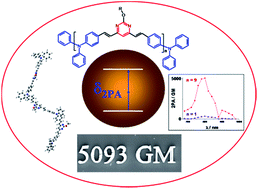
A comparative study of the linear and nonlinear optical properties of a novel triphenylamine–pyrimidine alternated oligomer and its corresponding V-shaped quadrupolar monomer is presented. Both chromophores strikingly exhibit the same spectral shape when considering their respective one- and two-photon absorption spectra. This effect was attributed to a weak interchromophore coupling within the oligomer which exhibits a highly distorted geometry resulting in a strong reduction of the effective conjugation length. The recursive implementation of nine monomers into a three-dimensional architecture leads however to a cooperative enhancement of the two-photon absorption (2PA) cross-section with a δMAX of 5093 GM at 800 nm. This very high 2PA ability has been oriented to improve the two-photon induced polymerization efficiency of a bicomponent photoinitiator system implying a hexaarylbiimidazole used as a H-abstractor and an aliphatic amine used as a H-donor. The photosensitizing mechanism is investigated and we clearly show that the intrinsic photoinitiation efficiency of the oligomer is increased by a factor 3 as compared to its corresponding monomer. We therefore demonstrate that such a two-photon sensitizing strategy leads to a synergy effect combining a higher photoinitiation reactivity and a very large two-photon absorption cross-section.

 Please wait while we load your content...
Please wait while we load your content...