Effective low temperature reduction of graphene oxide with vanadium(iii)†
Abstract
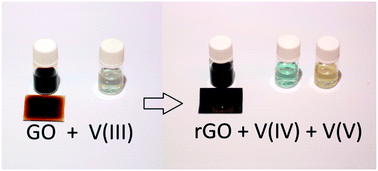
Reduction of graphene oxide (GO) with vanadium(III) trichloride under various reaction conditions has been investigated. The results show that V(III) can be used as an efficient reducing agent for GO in aqueous solutions at low concentrations and in moderate temperatures under ambient conditions. The IR spectroscopy and X-ray photoelectron spectroscopy (XPS) show that the structure of the vanadium-reduced material is similar to reduced graphene oxide prepared using TiCl3 or hydrazine as a reducing agent. The electrical conductivity of the material is also similar in all cases. However, on the basis of the XPS results, vanadium-based reduction does not leave significant reductant impurities in the product and does not lead to graphene substitution reactions. The success of vanadium(III) is somewhat surprising because the formal redox potential of the V(IV)/V(III) pair is rather anodic, but spectrophotometric studies of the reaction unambiguously showed that the process is a redox reaction. This method introduces a new facile graphene oxide reduction technique, which can be applied under ambient aqueous conditions.

 Please wait while we load your content...
Please wait while we load your content...