Lamellar nickel hydroxy-halides: anionic exchange synthesis, structural characterization and magnetic behavior†
Abstract
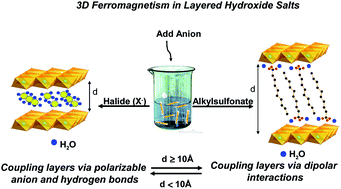
Nickel-layered hydroxy-halides LHS-Ni-X (X = Cl, Br, and I) have been prepared by exchange reactions conducted in an aqueous medium under an inert atmosphere starting from the parent nickel-layered hydroxyacetate. The latter was prepared by a hydrolysis reaction conducted in a polyol medium. IR and X-ray diffraction (XRD) studies show total exchange. These compounds exhibit a brucite-like structure with a turbostratic nature. Their interlamellar distance varies linearly with the radius of the halide anion in the range 7.9–8.7 Å while the hydroxyacetate interlamellar distance is 10.53 Å. In comparison with the acetate ion which replaces hydroxyl groups in the brucite-like layer, EXAFS and XRD investigations show that halide ions are intercalated into the interlayer space along with water molecules without any covalent bonding to the nickel ion. All compounds have similar structural features and can be considered as α-type nickel hydroxides, α-Ni(OH)2. These compounds exhibit a ferromagnetic character. The latter is discussed on the basis of the Drillon–Panissod model of ferromagnetic layers interacting via dipole interactions and taking into account the structural features established by XANES and XRD studies along with the intrinsic properties of the halide anions.

 Please wait while we load your content...
Please wait while we load your content...