Stabilizing cations in the backbones of conjugated polymers†
Abstract
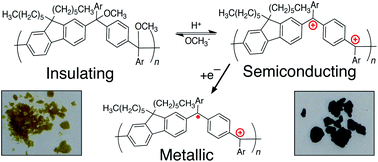
We synthesized a cross-conjugated polymer containing ketones in the backbone and converted it to a linearly conjugated, cationic polyarylmethine via a process we call “spinless doping” to create a new class of materials, conjugated polyions. This process involves activating the ketones with a Lewis acid and converting them to trivalent cations via the nucleophilic addition of electron-rich aryl moieties. Spinless doping lowers the optical band gap from 3.26 to 1.55 eV while leaving the intrinsic semiconductor properties of the polymer intact. Electrochemical reduction (traditional doping) further decreases the predicted gap to 1.18 eV and introduces radicals to form positive polarons; here, n-doping produces a p-doped polymer in its metallic state. Treatment with a nucleophile (NaOMe) converts the cationic polymer to a neutral, non-conjugated state, allowing the band gap to be tuned chemically, post-polymerization. The synthesis of these materials is carried out entirely without the use of Sn or Pd and relies on scalable Friedel–Crafts chemistry.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...