Novel design of organic donor–acceptor dyes without carboxylic acid anchoring groups for dye-sensitized solar cells†
Abstract
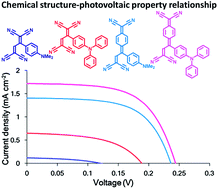
Organic donor–acceptor dyes, formed by a high-yielding [2 + 2] cycloaddition–retroelectrocyclisation process between aniline-substituted alkynes and tetracyanoethylene (TCNE) or 7,7,8,8-tetracyanoquinodimethane (TCNQ), were employed as novel photosensitizers without carboxylic acid anchoring groups in dye-sensitized solar cells (DSSCs). The efficient adsorption of the donor–acceptor dyes onto TiO2 was confirmed by UV-vis and IR spectroscopies. The photovoltaic performances of the DSSCs suggested that the triphenylamine derivatives 3 and 4 provide higher current densities (Jsc) as compared to the corresponding dimethylaniline counter molecules 1 and 2. This was mainly due to the excellent charge-separation efficiencies and lower charge-recombination rates of the triphenylamine moieties. It was also found that the devices sensitized by the TCNQ-adducted dyes 2 and 4 display open-circuit voltages (Voc) higher than those of the TCNE-adducted counter dyes 1 and 3. All these results were reasonably explained by the J–V curve fitting based on the equivalent-circuit model as well as the comparison between the absorption and incident-photon-to-current-conversion efficiency (IPCE) spectra.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...