Aggregation-enhanced emission in fluorophores containing pyridine and triphenylamine terminals: restricted molecular rotation and hydrogen-bond interaction†
Abstract
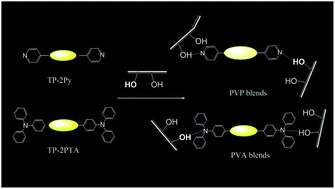
Restriction on molecular rotation of fluorophores reduces non-radiative decay channels and promotes strong fluorescence due to aggregation-enhanced emission (AEE) behavior. To evaluate the important role of restricted molecular rotation on AEE behavior, tetraphenylthiophene (TP) derivatives with two pyridine (Py) or two triphenylamine (TPA) terminals were synthesized and characterized to be AEE-active fluorophores. Because of the efficient hindered molecular rotation of the larger TPA terminals, TP-2TPA emitted with higher emission efficiency than TP-2Py with smaller Py terminals. In addition, Py and TPA terminals can serve as hydrogen-bond (H-bond) accepting groups to bind with H-bond donating hydroxyl groups in poly(vinyl phenol) (PVPh) and poly(vinyl alcohol) (PVA) to further reinforce rotational restriction on the TP-2Py and TP-2PTA fluorophores. TP-2Py and TP-2PTA were therefore blended with PVPh and PVA and the emissive properties of the resultant blends were characterized and compared with the unblended TP-2Py and TP-2PTA to emphasize the role of H-bond on restricted molecular rotation.

 Please wait while we load your content...
Please wait while we load your content...