Controlled release of lidocaine hydrochloride from polymerized drug-based deep-eutectic solvents†
Abstract
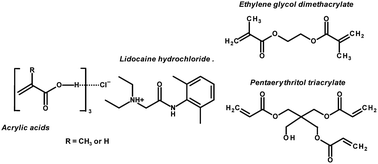
This work takes advantage of the transformation of lidocaine hydrochloride into deep-eutectic solvents (DESs) – ionic liquid analogues – to incorporate polymerizable counterparts into DESs, such that polymer–drug complexes are synthesized by free-radical frontal polymerization without the use of a solvent. DESs are formed through hydrogen bonding of an ammonium salt with a hydrogen-bond donor (HBD). It is demonstrated that lidocaine hydrochloride – as the ammonium salt – is able to form DESs with acrylic acid and methacrylic acid. The properties of DESs allow frontal polymerization in the bulk with full conversion achieved in a one-pot synthesis, yielding monoliths of polymers loaded with a high concentration of drug. In in vitro experiments, the sustained release of the drug takes place in a controlled manner triggered by the pH, ionic strength and solubility of the drug in the medium. Such control is owed to the swelling of polymers as well as to the specific interactions between the drug and the polymers already established in the DES precursor. Finally, it is noteworthy that different monomers (such as HBD) and crosslinkers can be used, thus expanding the possibilities of drug delivery systems for transdermal technologies by exploiting the DES chemistry.

 Please wait while we load your content...
Please wait while we load your content...