Influence of microporosity in SBA-15 on the release properties of anticancer drug dasatinib†
Abstract
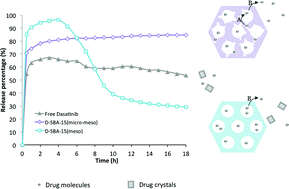
The release of the hydrophobic cancer drug dasatinib from two mesoporous silica materials as drug delivery vehicles has been studied. One material is a reference 2D-hexagonal SBA-15 with the typical bimodal pore system with ordered primary mesopores and disordered intrawall pores. The other material is a modified version of the same material where the intrawall porosity in the micropore regime has been selectively removed. Material characterization shows that, with the exception of the difference in intrawall porosity, the materials have identical properties. The drug dasatinib, a tyrosine kinase inhibitor, has been loaded, to the same extent, into the pores of both materials. The two materials give rise to very different release profiles of the drug. The presence of micropores leads to desired release properties: a high initial release of the drug, which is maintained over time. The lack of micropores also leads to a high initial release but followed by a rapid drop in the concentration of released drug, a consequence of its low solubility and hence crystallisation. We suggest that the presence of micropores in the carrier material, and the resultant kinetic release profile, leads to a stabilization of dasatinib in solution and to a sustained supersaturated level of the released drug. Our findings suggest that by controlling the mesoporous host with small variation in the textural properties, the kinetic release and crystallization behaviour of a drug can be altered. It is thus potentially possible to influence the drug post-release and thereby its bioavailability.

 Please wait while we load your content...
Please wait while we load your content...